Spectroscopy: items 42, 53, 55, 84, 172, 173
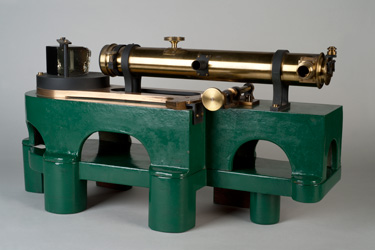
The line spectrum of an element, obtained by emission of light, was established as a means of identification by Bunsen and Kirchoff in 1859. The UV quartz prism spectrograph (item 172, 173) was manufactured by the well known scientific instrument firm of Adam Hilger Ltd., and was used for elemental analysis. The use of quartz for the dispersing prism gave access to lines in the ultraviolet region which would have been absorbed by a glass prism. This spectrograph recorded the spectrum on photographic plates. Such apparatus was still in use in undergraduate Chemistry laboratory courses in the early 1960s.
Small quantities of substances, either as solids on in solution, could be vaporized in flames and their emission spectra observed using simple hand spectroscopes, item 42 or more sophisticated prism spectroscopes, items 83 and 84, where the spectral lines were observed visually through a telescope and scanned by rotating the prism. Hand held spectroscopes such as item 42 could also be used to estimate the temperature of furnaces. One of the first new elements to be identified by spectroscopy was Thallium, discovered through observation of its prominent green spectral line by Crookes in 1861
Another means of vaporization was the spark struck between electrodes by means of the induction coil (item 53, 55) which generated high voltage AC potential from 12 V DC by rotating the handle of the apparatus. In this case, the sample was painted onto the electrodes.